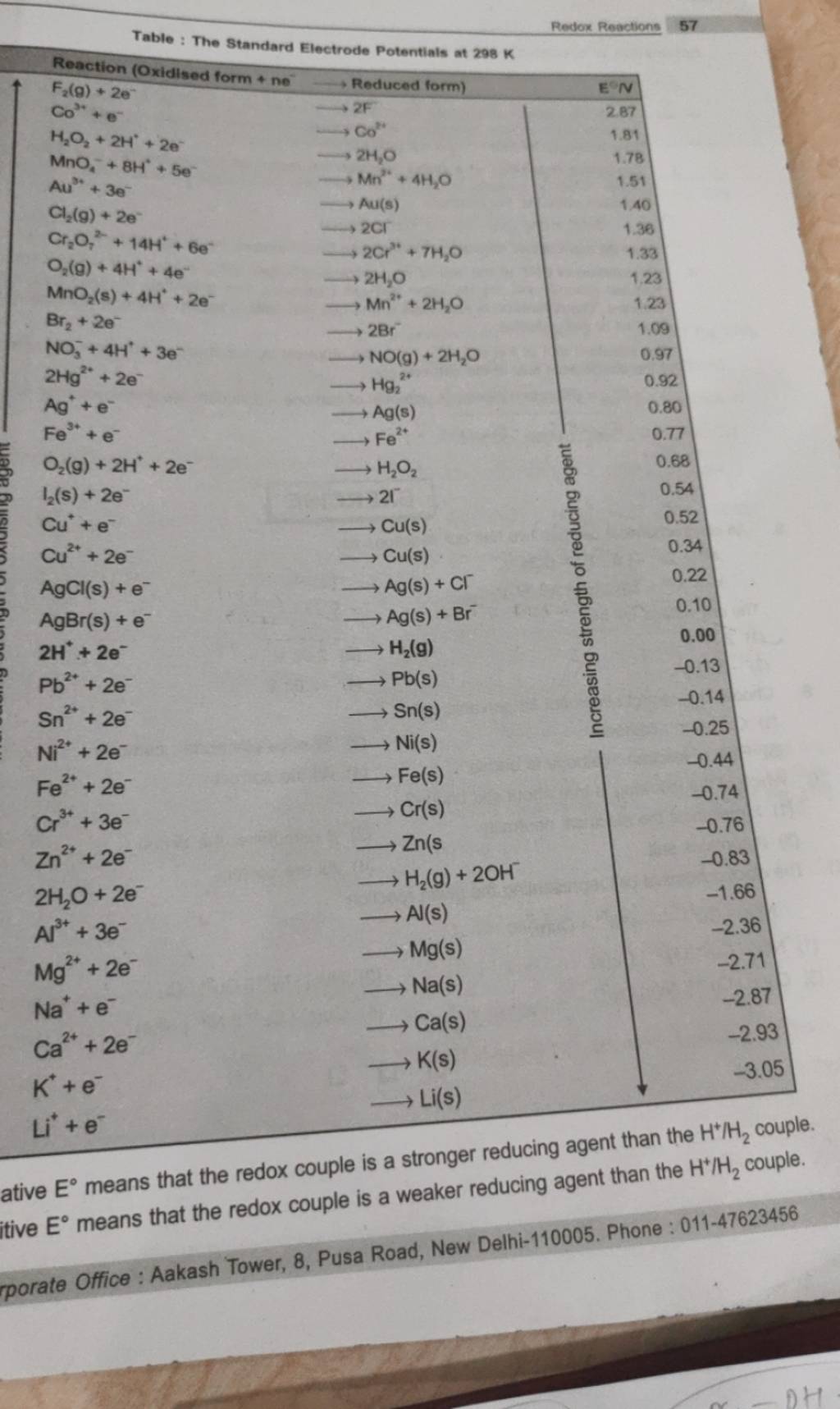
Standard Reduction Potential Table Ap Chem . Standard reduction potentials by value. The following table provides eo and eo ´ values for selected. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. Standard reduction potentials by element page id. The following table provides eo for selected reduction. Cu+ +e− → cu potential. Uc davis geowiki by university of california, davis. standard reduction potential measures the tendency for a given chemical species to be reduced. — from uc davis chem wiki (creative commons licence): since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table. The standard oxidation potential measures the tendency for a given chemical species to be oxidized as opposed to be reduced. 183 rows — p2:
from ar.inspiredpencil.com
The following table provides eo and eo ´ values for selected. standard reduction potential measures the tendency for a given chemical species to be reduced. Standard reduction potentials by element page id. 183 rows — p2: Uc davis geowiki by university of california, davis. The standard oxidation potential measures the tendency for a given chemical species to be oxidized as opposed to be reduced. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. The following table provides eo for selected reduction. Cu+ +e− → cu potential. — from uc davis chem wiki (creative commons licence):
Standard Reduction Potential Table
Standard Reduction Potential Table Ap Chem since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table. — from uc davis chem wiki (creative commons licence): Cu+ +e− → cu potential. Standard reduction potentials by element page id. The following table provides eo and eo ´ values for selected. Standard reduction potentials by value. The standard oxidation potential measures the tendency for a given chemical species to be oxidized as opposed to be reduced. 183 rows — p2: the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. standard reduction potential measures the tendency for a given chemical species to be reduced. since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table. Uc davis geowiki by university of california, davis. The following table provides eo for selected reduction.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Table Ap Chem Uc davis geowiki by university of california, davis. 183 rows — p2: Standard reduction potentials by element page id. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. The standard oxidation potential measures the tendency for a given chemical species to be oxidized as opposed. Standard Reduction Potential Table Ap Chem.
From pressbooks.uiowa.edu
Topic 11 Appendix A Standard Reduction Potentials at 25ºC CHEM 1120 Standard Reduction Potential Table Ap Chem The standard oxidation potential measures the tendency for a given chemical species to be oxidized as opposed to be reduced. 183 rows — p2: The following table provides eo and eo ´ values for selected. Uc davis geowiki by university of california, davis. the values of standard electrode potentials are given in the table in volts relative to. Standard Reduction Potential Table Ap Chem.
From www.chegg.com
Solved Standard Reduction Potential Table (at 25°C, 101kPa, Standard Reduction Potential Table Ap Chem The following table provides eo for selected reduction. since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. Cu+ +e− → cu potential. Standard reduction potentials by. Standard Reduction Potential Table Ap Chem.
From www.tpsearchtool.com
Appendix 4 Standard Reduction Potentials First Year General Chemistry Standard Reduction Potential Table Ap Chem Cu+ +e− → cu potential. standard reduction potential measures the tendency for a given chemical species to be reduced. since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table. 183 rows — p2: the values of standard electrode potentials are given in the table in volts relative. Standard Reduction Potential Table Ap Chem.
From www.studocu.com
Chapter 19 Recitation Problems CHEM 1310 Chapter 19 Use the standard Standard Reduction Potential Table Ap Chem the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. The standard oxidation potential measures the tendency for a given chemical species to be oxidized as opposed to be reduced. The following table provides eo and eo ´ values for selected. since copper is reduced in. Standard Reduction Potential Table Ap Chem.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Table Ap Chem the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. Cu+ +e− → cu potential. — from uc davis chem wiki (creative commons licence): The following table provides eo for selected reduction. since copper is reduced in the net ionic equation, we can use the. Standard Reduction Potential Table Ap Chem.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Table Ap Chem standard reduction potential measures the tendency for a given chemical species to be reduced. Cu+ +e− → cu potential. Uc davis geowiki by university of california, davis. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. The following table provides eo for selected reduction. Standard. Standard Reduction Potential Table Ap Chem.
From www.youtube.com
The diagonal rule of the standard reduction potential table (Part II Standard Reduction Potential Table Ap Chem The standard oxidation potential measures the tendency for a given chemical species to be oxidized as opposed to be reduced. standard reduction potential measures the tendency for a given chemical species to be reduced. The following table provides eo and eo ´ values for selected. Standard reduction potentials by value. Cu+ +e− → cu potential. the values of. Standard Reduction Potential Table Ap Chem.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Table Ap Chem Cu+ +e− → cu potential. Standard reduction potentials by element page id. The following table provides eo and eo ´ values for selected. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. since copper is reduced in the net ionic equation, we can use the. Standard Reduction Potential Table Ap Chem.
From ch302.cm.utexas.edu
Electrochemistry_Reduction_Potentials Standard Reduction Potential Table Ap Chem the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. The following table provides eo and eo ´ values for selected. Uc davis geowiki by university of california, davis. Standard reduction potentials by value. standard reduction potential measures the tendency for a given chemical species to. Standard Reduction Potential Table Ap Chem.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Reduction Potential Table Ap Chem standard reduction potential measures the tendency for a given chemical species to be reduced. Standard reduction potentials by value. Uc davis geowiki by university of california, davis. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. since copper is reduced in the net ionic. Standard Reduction Potential Table Ap Chem.
From apolympics.weebly.com
Triathlon Electrochemistry AP Chemistry Olympics Standard Reduction Potential Table Ap Chem — from uc davis chem wiki (creative commons licence): The standard oxidation potential measures the tendency for a given chemical species to be oxidized as opposed to be reduced. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. The following table provides eo for selected. Standard Reduction Potential Table Ap Chem.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Reduction Potential Table Ap Chem 183 rows — p2: — from uc davis chem wiki (creative commons licence): standard reduction potential measures the tendency for a given chemical species to be reduced. Uc davis geowiki by university of california, davis. The standard oxidation potential measures the tendency for a given chemical species to be oxidized as opposed to be reduced. the. Standard Reduction Potential Table Ap Chem.
From ecampusontario.pressbooks.pub
Appendix 4 Standard Reduction Potentials First Year General Chemistry Standard Reduction Potential Table Ap Chem The standard oxidation potential measures the tendency for a given chemical species to be oxidized as opposed to be reduced. Cu+ +e− → cu potential. Standard reduction potentials by element page id. The following table provides eo for selected reduction. Standard reduction potentials by value. standard reduction potential measures the tendency for a given chemical species to be reduced.. Standard Reduction Potential Table Ap Chem.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Table Ap Chem standard reduction potential measures the tendency for a given chemical species to be reduced. Cu+ +e− → cu potential. Standard reduction potentials by element page id. — from uc davis chem wiki (creative commons licence): Standard reduction potentials by value. Uc davis geowiki by university of california, davis. The following table provides eo and eo ´ values for. Standard Reduction Potential Table Ap Chem.
From pubs.acs.org
Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Standard Reduction Potential Table Ap Chem 183 rows — p2: Uc davis geowiki by university of california, davis. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table. Standard reduction potentials by. Standard Reduction Potential Table Ap Chem.
From f15.beauty
Standard Reduction Potential Table Standard Reduction Potential Table Ap Chem since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table. — from uc davis chem wiki (creative commons licence): 183 rows — p2: Standard reduction potentials by element page id. Cu+ +e− → cu potential. The following table provides eo and eo ´ values for selected. Standard reduction. Standard Reduction Potential Table Ap Chem.
From www.studocu.com
STANDARD REDUCTION POTENTIAL TABLE Chemical Engineering Studocu Standard Reduction Potential Table Ap Chem Standard reduction potentials by value. The following table provides eo for selected reduction. the values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for. The standard oxidation potential measures the tendency for a given chemical species to be oxidized as opposed to be reduced. Cu+ +e− → cu. Standard Reduction Potential Table Ap Chem.